


































































































39/99
\begin{frame}
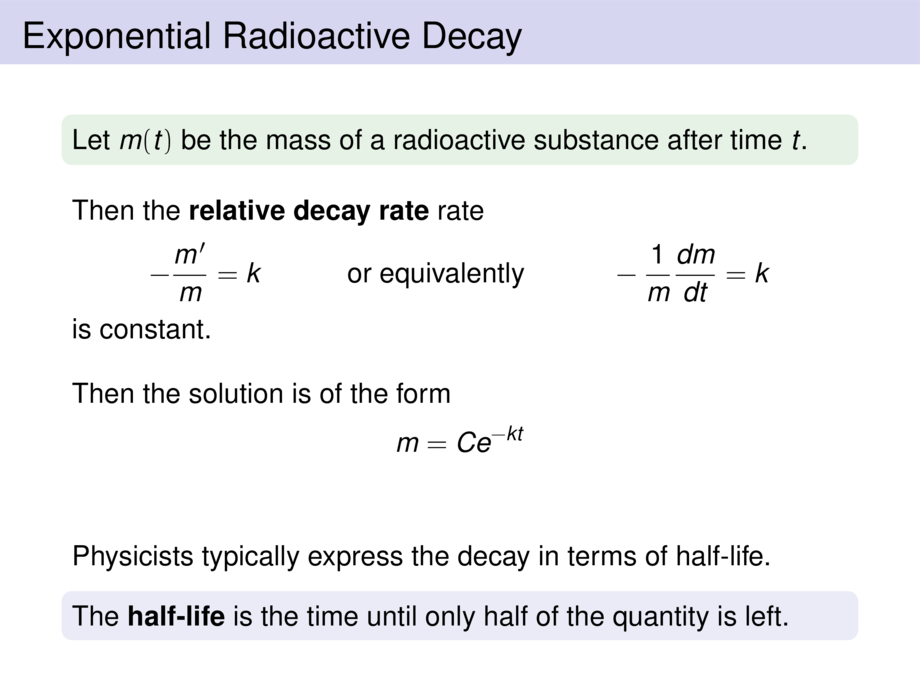
\frametitle{Exponential Radioactive Decay}
\begin{exampleblock}{}
The half-life of radium-226 is $1590$ years.
\begin{itemize}
\pause
\item We consider a sample of $100$mg.
\end{itemize}
\pause
Find a formula for the mass that remains after $t$ years.
\pause\medskip
We have:
\begin{talign}
&m(t) = m(0) \cdot e^{-kt} \\
&\mpause[1]{m(0) = 100} \\
&\mpause[2]{m(1590) = \frac{1}{2}\cdot 100 = 50 \mpause[3]{= 100 \cdot e^{-k\cdot 1590}}} \\
&\mpause[4]{e^{-k\cdot 1590} = \frac{1}{2} }
\mpause[5]{\;\;\implies\;\; -k\cdot 1590 = \ln \frac{1}{2} \mpause[6]{= \ln 1 - \ln 2 = -\ln 2} } \\
&\mpause[7]{k = \frac{\ln 2}{1590} }
\end{talign}
\pause\pause\pause\pause\pause\pause\pause\pause
Hence $m(t) = 100e^{-\frac{\ln 2}{1590} t} \mpause[1]{= 100 \left(\frac{1}{2}\right)^{\frac{t}{1590}} }$ is the mass after $t$ years.\hspace*{-10ex}
\end{exampleblock}
\end{frame}

